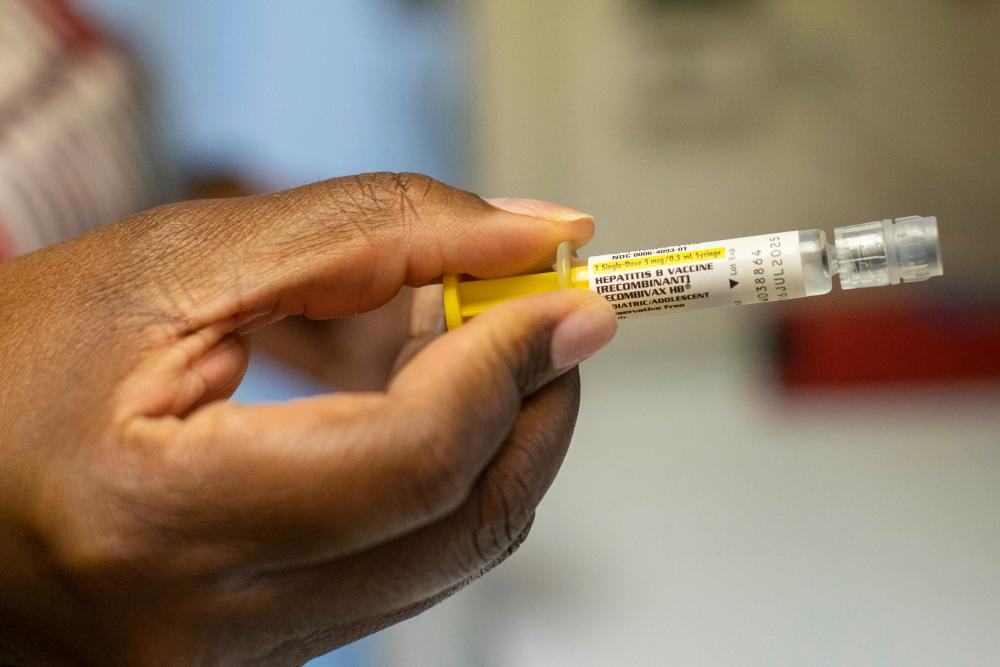
The Trump administration has indicated that it will fund a $1.6m study on hepatitis B vaccination of newborns in the west African country of Guinea-Bissau, where nearly one in five adults live with the virus – a move that researchers call “highly unethical” and “extremely risky”.
The news follows an official change in recommendations on hepatitis B vaccines at birth from the US Centers for Disease Control and Prevention (CDC), which called the shots an “individual” decision, despite decades of safe and effective vaccination and no evidence of harm. It is part of sweeping changes to childhood immunizations by the US health secretary, Robert F Kennedy Jr, which have global repercussions – including cutting funding for programs that bring vaccines to countries around the world.
“He has a fixed, immutable belief that vaccines cause harm,” said Paul Offit, director of the Vaccine Education Center and an attending physician at Children’s Hospital of Philadelphia. “He will do everything he can to try and prove that.”
The actions taken this year by Kennedy, a longtime anti-vaccine activist, have “a global impact”, said Elizabeth Jacobs, professor emerita at the University of Arizona and a founding member of the grassroots group Defend Public Health. “It is spreading like an infection all its own throughout the globe.”
Related: CDC ends recommendation for all US newborns to receive hepatitis B vaccine
Testing established vaccines in a country with high rates of hepatitis B and a fragile health system “reeks of a neocolonialist attitude” and risks expanding global mistrust of the US and science, said Gavin Yamey, professor of global health at the Duke Global Health Institute.
When Kennedy announced in June that the US would end funding to Gavi, the Vaccine Alliance, which has vaccinated more than 1.2 billion children and saved an estimated 20.6 million lives, he sent shock waves through global health – and he cited an unusual study from 2018 to justify the action.
The study made an alarming claim: the diphtheria-tetanus-pertussis (DTP) vaccine caused death in young girls in Guinea-Bissau. It was published by a group of Danish researchers, including a married couple named Peter Aaby and Christine Stabell Benn.
But when Kennedy made his announcement in 2025, he did not mention a 2022 paper from some of the same authors on the same topic finding completely different results, essentially nullifying the first study.
“We did not find that early-DTP was associated with increased female mortality as found in a previous study,” the researchers wrote.
It is one example of their questionable research, which has drawn criticism from other researchers and journalists examining their findings.
Now those same researchers will be the ones carrying out the new study on hepatitis B vaccination in Guinea-Bissau. US funding will go to the Bandim Health Project, led by Aaby and Stabell Benn, at the University of Southern Denmark.
Aaby and Stabell Benn did not respond to the Guardian’s inquiries about the details of the five-year study, set to begin in early 2026.
Babies in the randomized, controlled trial will or will not receive the hepatitis B vaccine at birth. Researchers will then compare early-life mortality, illness and development between the groups, according to the award announcement from the CDC.
The World Health Organization recommends giving the vaccine to all babies at birth, but Guinea-Bissau has struggled to roll out the shots to every newborn, instead recommending the dose at six weeks of age. The country has pledged to fill that gap and plans on recommending hepatitis B vaccines to all newborns in 2027.
It is a major breach of scientific ethics to withhold an intervention that has been proven safe and effective. “It’s highly unethical to choose to give a vaccine to some children but not others,” Offit said.
Yamey noted: “There’s already an RCT [randomized, controlled trial] showing superior outcomes with the birth dose, so why is another one needed?”
The study does not appear to be looking at whether the vaccine is more effective at birth, which Jacobs said was “concerning” as “nowhere in this do they say that they’re going to study the efficacy of the vaccine itself.”
Instead, the trial will examine whether there are “overall health effects” – not specific outcomes, such as preventing infection from the virus – when the shot is given at birth, according to the Bandim Health Project.
“This announcement has set alarm bells ringing in the global health community,” said Martin McKee, professor of European public health at the London School of Hygiene and Tropical Medicine, calling it a symptom of “a policy desperately searching for evidence”.
“It is not clear what the research question is. It seems to be about the safety of the vaccine rather than its effectiveness, but both are already well-established, and to undertake such a study in a population where almost one in five of the adult population has a marker of infection seems extremely risky,” McKee said.
He also questioned whether participants could truly give informed consent, given the ethical concerns about how the study is being conducted.
In a recent survey, about 18% of Bissau-Guinean adults had hepatitis B, a virus that can lead to cirrhosis or liver cancer, especially among young children. If a baby is infected in the first year of life, there is a 90% chance they will develop cirrhosis or liver cancer; between the ages of one and five, there is a 25% chance. Among adults, about 5% have a chronic infection.
In a recent study of toddlers in Guinea-Bissau, about 11.2% already had hepatitis B infection, which means not enough babies are getting the shots, said Andrew Pollard, professor of paediatric infection and immunity and director of the Oxford Vaccine Group at the University of Oxford. Across sub-Saharan Africa, only about 17% of babies receive the recommended birth dose, he added.
“The priority should be to increase vaccination with the birth dose of hepatitis B vaccine and protect more babies from the risk posed by this virus,” Pollard said.
In the US, recommending the vaccine at birth to all babies – not just those who appeared to be at risk of infection – caused rates among children to drop precipitously, from 20,000 to about 20 a year.
“We virtually eliminated hepatitis B in children less than 10,” Offit said. Children may be infected at birth, but they may also come into contact with other children and adults carrying the virus – which can remain infectious on surfaces for up to a week.
The experts voiced concerns about how the study would take place. It is unusual for a trial like this to take place in Guinea-Bissau instead of the United States or Denmark, they said.
“Why on Earth is this study happening in a high-endemic setting where the birth dose matters the most?” Yamey asked.
In Denmark, where only three in 1,000 people have the virus, the shot is not currently recommended at birth either, which means the same study could be conducted there. Denmark also has health registries, making it easier to access complete medical records. Instead, by working in a country with precarious healthcare and high rates of the illness, studies like this may lead to “expanding distrust in global public health”, Jacobs said.
The US canceled much of its global aid and research earlier this year, Jacobs said.
“In the face of the US canceling all this funding for vulnerable countries, and then it’s still going to pay for this research to be done – that is really worrying,” she said. “It seems to say we don’t value your lives enough to continue to provide support overall, but we won’t hesitate to experiment with your population.”
The study is single-blinded, which means the patients will not know who got the vaccine and who didn’t, but the research team will – which can affect the way they collect and interpret the data. “This means they can stamp their own biases on the results,” Yamey said. And the endpoints – “overall health effects” – are “very squishy”, which leaves the results vulnerable to manipulation, Jacobs said.
Henrik Støvring, a professor of statistics and pharmacometrics at Aarhus University who co-wrote about red flags in Bandim Health Project research for the journal Vaccine this month, said “broad hypotheses like these carry a high risk of false positive findings, and in general the research group has previously been reluctant to use appropriate statistical methods to curb such a risk.”
“I think conflicts of interests are always an issue when the donor so explicitly seeks out a research group and funds a study,” Støvring said.
The Danish journalist Gunver Lystbæk Vestergård has also written about major issues with research conducted by Aaby and Stabell Benn.
After the CDC sparked outrage by changing the recommendation for hepatitis B vaccination with no evidence, Jacobs said, “they’re now funding this to try to give themselves cover for having done that.”
“Because Robert F Kennedy Jr is an anti-vaccine zealot, he will somehow contort that study to look like the hepatitis B birth dose causes harm” or that it is better to delay the shots, Offit said.
Scientists, doctors and medical organizations are speaking out against Kennedy, he said, but “this is a political problem, and it requires a political solution.”
In the meantime, children will bear the brunt of these decisions, Offit continued: “This breaks my heart. It really does. It’s hard to sleep knowing that children are constantly being put in harm’s way by the administration.”